2022-02-22
The effects of water purity on removal of hydrophobic substances from solid surfaces without surfactants

Andriani Tsompou and Vitaly Kocherbitov
Department of Biomedical Science and Biofilms research center for Biointerfaces, Malmö University.
To reduce greywater pollution and environmental pressure on aquatic ecosystems, it is urgent to investigate new washing and cleaning methods. This new study shows that washing with purified water only, can be almost as efficient as using traditional washing with detergents. This means a substantial decrease of the amount of detergents in cleaning and washing applications may be possible.
Synthetic detergents have been around for more than 100 years and they are part of the household routine for people worldwide. The use of detergents is, however, a well-known source of water pollution. More than 60% of the total surfactant production enters the aquatic environment resulting in water and environmental pollution. Another drawback of the use of surfactants is their possible negative effect on human health, as they may cause allergic reactions, skin irritation and inflammation. In fact, 2.5% of washing detergents remain on clothes after traditional washing.
The idea to decrease or shies the use of detergents in the cleaning process has been around for some time. Using natural surfactants, degassed water or a combination of natural and synthetic surfactants have been studied for a long period of time. We present a different idea, of using ultra-pure water, as an alternative way of washing and cleaning without detergents. This could potentially lead to a more environmentally friendly method of cleaning. Making possible a substantial decrease of the amount of detergents in cleaning and washing applications. Although it was already commonly accepted that washing with purified water was highly efficient, the mechanism of this process was never investigated. Because of this, the method is still not broadly used. This study explains mechanisms behind this process. We suggest that the absence of ions in purified water grades increases the electrostatic repulsion and facilitates the removal of dirt from surfaces.
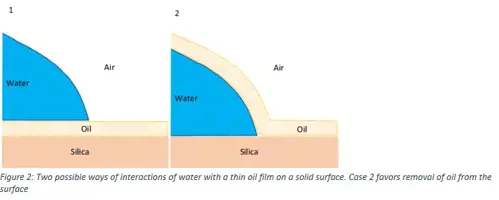
The efficiency of pure water in detergent-free cleaning procedure using a method called Quartz Crystal Microbalance with Dissipation (QCM-D) monitoring was also examined. The results (Figure 1) indicate that cleaning using detergent (SDS) is most efficient, followed by MilliQ water and DIRO. In addition, the study of the properties of the interfaces, gave the possibility to understand how the hydrophobic substance is removed from the surface.
Having a closer look on methodology, Contact angle photographic results (Figure 3) indicate that purified water grades facilitate the roll-up mechanism (Figure 2), by forming droplets that can be easily removed with mechanical energy (contact angle between 90o-180o). In the case of non-purified water grades, where the contact angle is lower, mechanical energy alone cannot wash away the dirt completely and a certain amount will remain attached.